Test Material Preparation and Submission
This guideline is prepared to help you understand the importance of proper "sample preparation" for the different tests performed for the biological evaluation of materials.
It must be noted that testing is to be performed on the final product, or on representative samples taken from the final product or from materials processed in the same manner as the final product.
Submitting proper samples will go a long way in ensuring that you get the proper test reports in time.
We strongly advise you to visit us and discuss your testing requirements and get first hand information on the sample requirements for the selected tests; especially if you plan to submit the test reports to any regulatory agency.
GENERAL INFORMATION:
We conduct tests on the samples submitted by you and the test results provide results for the test samples only. We do not certify your batch or product quality.
Our test reports will specify the test material/sample code as mentioned by you in the test request forms. Kindly ensure that you have specified the proper sample code that will reflect your end use. If you are submitting the test reports to a regulatory agency, ensure that the test sample codes will provide unambiguous traceability to your material/product. We will not accept requests for change of test sample codes or other details such as name of company etc. on the test report after testing.
General Guidelines:
Dos
1. Contact the lab and understand the specific requirements beforehand
2. Follow the specific instructions on each test request form
3. Submit samples in separately marked packets for different tests
4. When replicates are required, pack the samples in separate individual packets
5. Label the packets correctly
6. Indicate the sterilization status- whether sterile and method of sterilization
7. Indicate clearly, special storage conditions if any for your sample
8. Do indicate shelf life of samples when the sample is unstable or has a limited shelf life only
9. Do submit appropriate CONTROL samples, if required as part of the study/test.
10. If test materials are to be archived then a special request need to be given. Test materials will be archived for a maximum of 3 years.
Don'ts
1. Do not submit samples with hazardous / infectious/ radioactive content.
2. Don't have sharp and pointed edges for your samples. Remove any burrs or sharp edges from the sample pieces.
3. Don't submit samples before prior intimation to the customer service cell, either by post or in person.
4. Don't mix samples for different tests together. Pack the samples for different tests separately.
5. Do not use staplers or pins on sterile packets. If sending by post/courier, ensure that there is no damage due to stapling by the courier agency.
Test Material Preparation
IN VITROCYTOTOXICITY (ISO 10993)
Direct / Indirect Contact
Shape and Size - Test material must have a flat surface & with one side having a surface area of approx 0.1 cm2/ 0.3cm2
OR
Test material with 4mm diameter disc
Quantity - 6 numbers of the material in any of the above specification - individually packed and in sterile form.
Test on Extract
Kindly specify the total surface area and weight of the sample in this case. Please stick on to any of the following specifications as per the thickness & nature of your test material.
Total surface area = 6 cm2
Total surface area = 3 cm2
Total surface area = 3 cm2
Total surface area = 1.25 cm2
IRRITATION /ACUTE SYSTEMIC TOXICITY/ GENOTOXICITY (ISO 10993)
What is the thickness (T) of your material?
Based on the thickness please select the option that suits your material from below
|
T<0.5 mm Film, sheet, tubing wall |
T=0.5 to 1mm Tubing wall, slab, small moulded items |
T >1 mm Large moulded items |
T >1 mm Elastomeric closures |
Irregularly shaped solid devices Powder, pellets, foam, nonabsorbent moulded items |
|
• 20 strips with the below dimensions in one packet • Such 4 packets 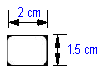
|
• 20 strips with the below dimensions in one packet • Such 4 packets 
|
• 20 strips with the below dimensions in one packet • Such 4 packets 
|
• 5 strips with the below dimensions in one packet • Such 4 packets 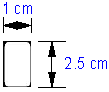
|
• 2g in one packets • Such 8 packets |
SENSITIZATION
What is the thickness (T) of your material?
Based on the thickness please select the option that suits your material from below
|
T<0.5 mm Film, sheet, tubing wall |
T=0.5 to 1mm Tubing wall, slab, small moulded items |
T >1 mm Large moulded items |
T >1 mm Elastomeric closures |
Irregularly shaped solid devices Powder, pellets, foam, nonabsorbent moulded items |
T< 0.5 cm Materials to be tested without modification, Solid material |
|
• 20 strips with the below dimensions in one packet • Such 22 packets 
|
• 20 strips with the below dimensions in one packet • Such 22 packets 
|
• 20 strips with the below dimensions in one packet • Such 22 packets 
|
• 5 strips with the below dimensions in one packet • Such 22 packets 
|
• 2g in one packets • Such 44 packets |
• 10 strips with the below dimensions in one packet • Such 11 packets 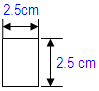
|
PYROGEN
|
T<0.5 mm Film, sheet, tubing wall |
T=0.5 to 1mm Tubing wall, slab, small moulded items |
T >1 mm Large moulded items |
T >1 mm Elastomeric closures |
Irregularly shaped solid devices Powder, pellets, foam, nonabsorbent moulded items |
Membranes Irregularly shaped porous devices |
|
• 3 strips with the below dimensions in one packet • Such 20 packets 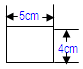
|
• 3 strips with the below dimensions in one packet • Such 20 packets 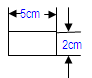
|
• 3 strips with the below dimensions in one packet • Such 20 packets 
|
• 5 strips with the below dimensions in one packet • Such 22 packets 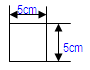
|
• 2g in one packets • Such 40 packets |
• 2g in one packets • Such 20 packets |
IMPLANTATION (ISO 10993)
| Implantation in muscle | Implantation in subcutaneous tissue | Implantation in bone |
1 mm width x 10mm length (rounded edges and the ends finished to full radius)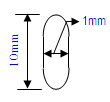
|
10mm to 12mm in diameter x 0.3mm to 1mm thickness
OR 1.5mm diameter x 5mm length smooth radius ends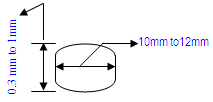
OR 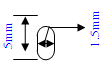
|
Cylindrical implants 2mm diameter X 6 mm in length
OR 2mm to 4.5 mm orthopaedic bone screw type implants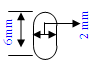
OR 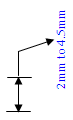
|
HEMOLYTIC PROPERTY
| Film, sheet, tubing wall | Powders / Irregular shaped Materials |
|
• 10 strips with the below dimensions per packet • Such 6 packets AND • 7 strips with the below dimensions per packet• Such 6 packets 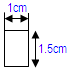
|
• 2g per packet • 6 packets AND • 1.4g per packet• 6 packets |
